What Is The Molar Mass Of Pb No3 2
sandbardeewhy
Dec 04, 2025 · 11 min read
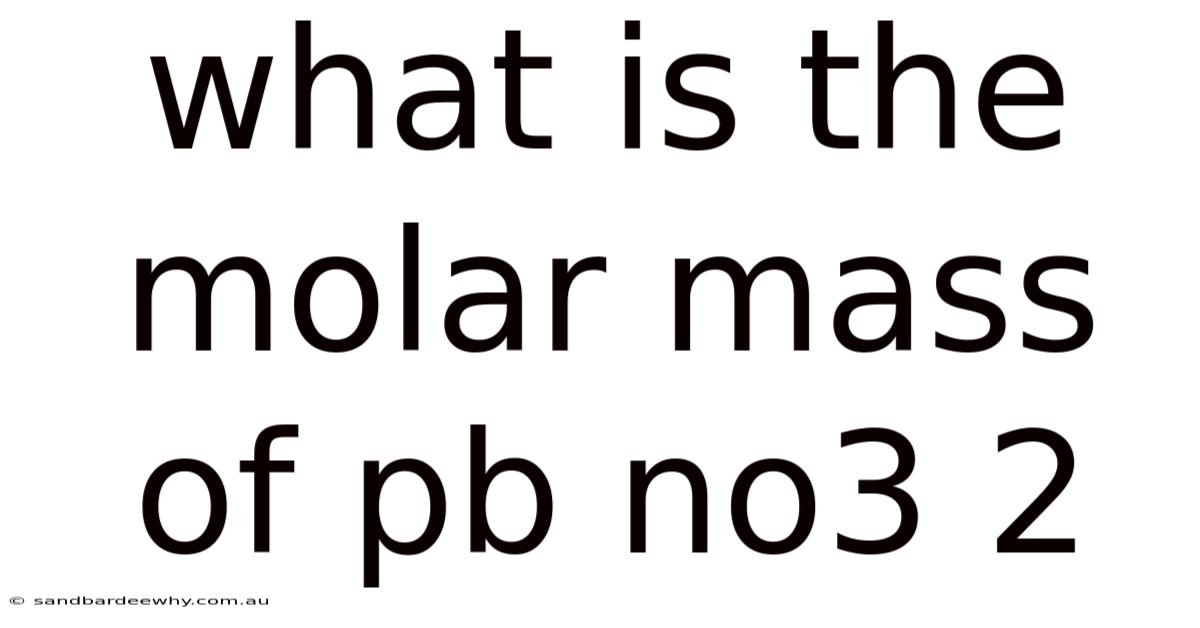
Table of Contents
Imagine you're in a chemistry lab, carefully weighing out a compound for a crucial experiment. You need lead(II) nitrate, or Pb(NO₃)₂, and the recipe calls for a specific number of moles. But how do you translate that into grams? This is where the concept of molar mass becomes indispensable. The molar mass acts as a conversion factor, bridging the gap between the abstract world of moles and the tangible reality of grams, allowing you to accurately measure and manipulate chemical substances.
Perhaps you're working on a research project involving lead compounds, or maybe you're simply a student tackling a stoichiometry problem. In either case, knowing the molar mass of Pb(NO₃)₂ is essential. It's a fundamental piece of information that unlocks the door to countless calculations and experimental procedures. This article will guide you through understanding what molar mass is, how to calculate it, and why it's so vital in chemistry, using Pb(NO₃)₂ as a practical example.
Main Subheading: Understanding Molar Mass
Molar mass is a cornerstone concept in chemistry, acting as a bridge between the microscopic world of atoms and molecules and the macroscopic world we interact with daily. It's the mass of one mole of a substance, expressed in grams per mole (g/mol). A mole, in turn, is defined as 6.022 x 10²³ entities (atoms, molecules, ions, etc.), a number known as Avogadro's constant. Essentially, molar mass tells you how much one "packet" of 6.022 x 10²³ particles of a particular substance weighs.
Think of it like buying eggs. You don't usually buy individual eggs; you buy them by the dozen. A "dozen" is a convenient unit for counting eggs, just like a "mole" is a convenient unit for counting atoms and molecules. The molar mass is like knowing the weight of a dozen eggs – it allows you to quickly determine the mass of any given number of "dozens" (or in the case of chemistry, moles) of that substance. For instance, if you know the molar mass of water (H₂O) is approximately 18 g/mol, you know that 6.022 x 10²³ water molecules weigh 18 grams. This allows chemists to accurately measure out the correct amounts of reactants needed for chemical reactions.
Comprehensive Overview
To fully grasp the significance of molar mass, it's helpful to delve into its definitions, historical context, and underlying scientific principles.
Definition and Formula: As mentioned earlier, molar mass is the mass of one mole of a substance, expressed in grams per mole (g/mol). It is numerically equivalent to the atomic or molecular weight of the substance expressed in atomic mass units (amu). The formula for calculating molar mass is relatively simple: sum the atomic masses of all the atoms in the chemical formula. This information is readily available on the periodic table.
Scientific Foundations: The concept of molar mass is rooted in the atomic theory, which states that all matter is composed of atoms. Each element has a unique atomic mass, which is the average mass of its isotopes, weighted by their natural abundance. These atomic masses are the foundation upon which molar masses are built. The mole concept, formalized by Avogadro's constant, provides the crucial link between atomic mass and macroscopic quantities. Without a standardized way to count atoms and molecules, it would be impossible to accurately predict the outcomes of chemical reactions.
Historical Context: The development of the mole concept and the determination of accurate atomic masses were significant achievements in the history of chemistry. Early chemists like John Dalton recognized that elements combine in fixed ratios to form compounds, leading to the development of the law of definite proportions. However, it wasn't until the work of Stanislao Cannizzaro and others in the 19th century that a consistent and accurate scale of atomic weights was established. The subsequent determination of Avogadro's number allowed scientists to connect atomic masses to measurable quantities of substances, paving the way for the widespread use of molar mass in chemical calculations.
Calculating Molar Mass of Pb(NO₃)₂: Now, let's apply these principles to calculate the molar mass of lead(II) nitrate, Pb(NO₃)₂.
-
Identify the elements: Pb, N, and O.
-
Find the atomic masses: Look up the atomic masses of each element on the periodic table:
- Pb (Lead): 207.2 g/mol
- N (Nitrogen): 14.01 g/mol
- O (Oxygen): 16.00 g/mol
-
Account for subscripts: Note the subscripts in the chemical formula Pb(NO₃)₂. This indicates that there is 1 lead atom, 2 nitrogen atoms (1 x 2), and 6 oxygen atoms (3 x 2).
-
Multiply and sum: Multiply the atomic mass of each element by the number of atoms of that element in the formula, and then sum the results:
Molar mass of Pb(NO₃)₂ = (1 x 207.2 g/mol) + (2 x 14.01 g/mol) + (6 x 16.00 g/mol) Molar mass of Pb(NO₃)₂ = 207.2 g/mol + 28.02 g/mol + 96.00 g/mol Molar mass of Pb(NO₃)₂ = 331.22 g/mol
Therefore, the molar mass of Pb(NO₃)₂ is approximately 331.22 g/mol.
Significance of Molar Mass: The molar mass is not just a number; it's a critical tool in various chemical applications.
- Stoichiometry: It allows chemists to predict the amounts of reactants and products involved in chemical reactions. By knowing the molar masses of the substances, you can convert between mass, moles, and number of particles, enabling you to calculate theoretical yields and determine limiting reactants.
- Solution Preparation: In preparing solutions of specific concentrations, the molar mass is essential for calculating the mass of solute needed to dissolve in a given volume of solvent. For instance, if you need a 1 M solution of Pb(NO₃)₂, you would dissolve 331.22 grams of Pb(NO₃)₂ in enough water to make 1 liter of solution.
- Elemental Analysis: Molar mass is used in elemental analysis to determine the percentage composition of elements in a compound. By comparing the experimentally determined mass percentages with the theoretical values calculated from the molar mass, you can confirm the purity and identity of a substance.
- Research and Development: In research settings, accurate molar mass values are crucial for characterizing new compounds, understanding reaction mechanisms, and developing new materials.
Trends and Latest Developments
While the fundamental concept of molar mass remains constant, its application and the methods used to determine it are continually evolving. Here are some trends and recent developments related to molar mass:
- Improved Measurement Techniques: Advances in mass spectrometry have enabled more accurate and precise determination of atomic masses and, consequently, molar masses. High-resolution mass spectrometers can distinguish between isotopes with very small mass differences, leading to more reliable molar mass values.
- Computational Chemistry: Computational methods are increasingly used to predict and refine molar mass values. Quantum chemical calculations can provide accurate estimates of atomic and molecular weights, especially for complex molecules where experimental determination is challenging.
- Isotopic Analysis: Isotopic analysis is gaining importance in various fields, including environmental science, geochemistry, and forensics. By measuring the ratios of different isotopes in a sample, scientists can gain insights into its origin, age, and history. Molar mass, when considered in the context of isotopic composition, provides valuable information for these analyses.
- Nanomaterials: The field of nanomaterials is heavily reliant on accurate molar mass determination. The properties of nanomaterials are often size-dependent, and precise knowledge of their molar mass is crucial for controlling their synthesis and characterizing their behavior.
- Data Availability: Online databases and software tools are making it easier than ever to access and calculate molar masses. These resources provide up-to-date atomic mass values and automated calculators that simplify the process of determining molar masses for complex compounds.
These trends highlight the ongoing importance of molar mass in various scientific disciplines and the continuous efforts to improve its accuracy and accessibility. The integration of advanced measurement techniques, computational methods, and data resources is transforming the way molar mass is used and understood.
Tips and Expert Advice
Calculating and using molar mass effectively requires attention to detail and a solid understanding of chemical principles. Here are some practical tips and expert advice to help you master this essential skill:
-
Use a Reliable Periodic Table: Always use a reputable and up-to-date periodic table to find the atomic masses of the elements. Different periodic tables may have slight variations in the reported atomic masses, so it's important to use a consistent source. Online databases such as the NIST (National Institute of Standards and Technology) Chemistry WebBook are excellent resources for accurate atomic mass values.
-
Pay Attention to Subscripts and Parentheses: When calculating the molar mass of a compound with subscripts and parentheses, carefully account for the number of atoms of each element. Remember that subscripts outside parentheses apply to all the elements inside the parentheses. For example, in Pb(NO₃)₂, the subscript 2 applies to both nitrogen and oxygen, meaning there are two nitrogen atoms and six oxygen atoms.
-
Use Appropriate Units: Always include the correct units (g/mol) when reporting molar mass values. This helps to avoid confusion and ensures that your calculations are dimensionally consistent.
-
Check Your Work: Double-check your calculations to ensure that you have correctly summed the atomic masses and accounted for all the atoms in the formula. A small error in the molar mass can lead to significant errors in subsequent calculations.
-
Understand Significant Figures: Pay attention to significant figures when using molar mass in calculations. The number of significant figures in your final answer should be consistent with the least precise measurement used in the calculation. For example, if you are using a molar mass value with four significant figures and a mass measurement with three significant figures, your final answer should be rounded to three significant figures.
-
Consider Hydrates: If you are working with a hydrated compound (a compound that contains water molecules), be sure to include the mass of the water molecules in the molar mass calculation. For example, copper(II) sulfate pentahydrate (CuSO₄·5H₂O) contains five water molecules per formula unit. To calculate its molar mass, you would add the mass of five water molecules (5 x 18.02 g/mol) to the molar mass of CuSO₄.
-
Practice Regularly: The best way to become proficient in calculating and using molar mass is to practice regularly. Work through a variety of examples, including simple compounds, complex molecules, and hydrated compounds. The more you practice, the more confident you will become in your ability to apply this essential skill.
-
Use Online Tools: Take advantage of online molar mass calculators and resources to simplify the process. These tools can quickly calculate the molar mass of a compound based on its chemical formula, saving you time and effort. However, it's still important to understand the underlying principles and be able to perform the calculations manually.
-
Apply Molar Mass in Real-World Problems: To truly understand the significance of molar mass, try applying it to real-world problems. For example, calculate the mass of a reactant needed to produce a specific amount of product in a chemical reaction, or determine the concentration of a solution based on the mass of solute dissolved.
By following these tips and expert advice, you can enhance your understanding of molar mass and improve your ability to apply it effectively in various chemical applications.
FAQ
Q: Why is molar mass important in chemistry?
A: Molar mass is crucial because it allows chemists to convert between mass and moles, enabling accurate measurements and predictions in chemical reactions and solution preparation. It links the microscopic world of atoms and molecules to the macroscopic world we can measure.
Q: How is molar mass different from atomic mass?
A: Atomic mass refers to the mass of a single atom of an element, typically expressed in atomic mass units (amu). Molar mass, on the other hand, is the mass of one mole (6.022 x 10²³ particles) of a substance, expressed in grams per mole (g/mol). The numerical values are the same, but the units differ.
Q: Can the molar mass of a compound be a fraction?
A: Yes, the molar mass of a compound is often a fraction because it's based on the average atomic masses of the elements, which are weighted averages of the masses of their isotopes.
Q: Where can I find reliable atomic mass values?
A: Reputable periodic tables, chemistry textbooks, and online databases like the NIST Chemistry WebBook provide reliable atomic mass values.
Q: What is the unit for molar mass?
A: The unit for molar mass is grams per mole (g/mol).
Conclusion
In summary, the molar mass of Pb(NO₃)₂ is approximately 331.22 g/mol. Understanding the concept of molar mass, its calculation, and its applications is fundamental to success in chemistry. From stoichiometry and solution preparation to elemental analysis and research, molar mass plays a vital role in connecting the microscopic and macroscopic worlds. By mastering this essential concept, you gain a powerful tool for understanding and manipulating chemical substances.
Now that you have a solid grasp of molar mass, take the next step! Try calculating the molar masses of other compounds, practice using molar mass in stoichiometric calculations, or explore online resources for further learning. Your journey into the world of chemistry has just begun, and with a firm understanding of molar mass, you're well-equipped to tackle the challenges and explore the wonders that lie ahead.
Latest Posts
Latest Posts
-
What Are Seven Elements Of Art
Dec 04, 2025
-
Science Word That Starts With E
Dec 04, 2025
-
1 5 6 As An Improper Fraction
Dec 04, 2025
-
How Many Liquid Ounces In A Pint
Dec 04, 2025
-
Calpurnia From To Kill A Mockingbird
Dec 04, 2025
Related Post
Thank you for visiting our website which covers about What Is The Molar Mass Of Pb No3 2 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.