Avogadro's Number Is Equal To 6.02x1023
sandbardeewhy
Nov 28, 2025 · 10 min read
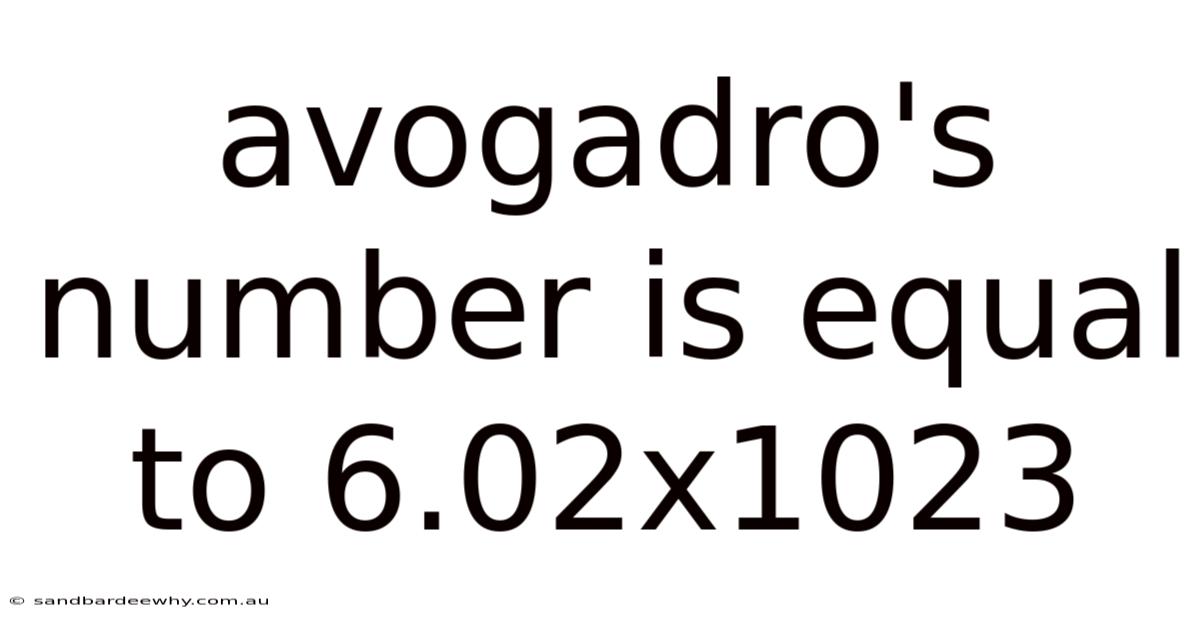
Table of Contents
Imagine you're baking a cake, not just any cake, but one that needs to be absolutely perfect in every way. You wouldn't just throw ingredients together haphazardly, would you? No, you'd carefully measure each component to ensure the right balance of flavors and textures. In chemistry, Avogadro's number plays a similar role, acting as a precise measurement tool that allows us to understand the fundamental relationships between the microscopic world of atoms and molecules and the macroscopic world we experience every day.
Think about trying to count grains of sand on a beach. It would be an impossible task, right? Similarly, atoms and molecules are so incredibly tiny that counting them individually is out of the question. This is where Avogadro's number, 6.02 x 10^23, comes to the rescue. It provides a bridge, linking the number of particles (atoms, molecules, ions, etc.) to the amount of substance, measured in moles. Just as a "dozen" always means 12, a mole always means 6.02 x 10^23 entities. This constant is crucial for chemists, enabling them to perform quantitative analysis, predict reaction outcomes, and synthesize new materials with accuracy and precision.
Unveiling Avogadro's Number: A Cornerstone of Chemistry
Avogadro's number, denoted as NA, is one of the most fundamental constants in chemistry, linking the macroscopic and microscopic worlds. It represents the number of constituent particles (usually atoms or molecules) that are contained in the amount of substance given by one mole. Its value is approximately 6.02214076 × 10^23. This number allows chemists to perform accurate quantitative analyses by connecting the mole, a unit of amount, to a specific number of particles.
The concept behind Avogadro's number wasn't conceived overnight; it evolved over time through the work of numerous scientists. While the number is named in honor of Italian scientist Amedeo Avogadro, he did not actually determine its value. Avogadro proposed in 1811 that equal volumes of gases at the same temperature and pressure contain the same number of molecules, regardless of their chemical nature. This hypothesis, known as Avogadro's Law, laid the groundwork for understanding the relationship between the number of particles and the volume of a gas.
The actual determination of Avogadro's number came later, primarily through the work of scientists like Josef Loschmidt, who made the first reasonable estimate of the size of molecules in 1865. Jean Perrin, in the early 20th century, conducted experiments on Brownian motion and used his findings to calculate NA more accurately. Perrin's work was so significant that it provided strong evidence for the existence of atoms and molecules, solidifying the atomic theory of matter. His meticulous experiments and calculations earned him the Nobel Prize in Physics in 1926.
The precise determination of Avogadro's number has been refined over the years using various methods. One of the earliest methods involved electrolysis, where the amount of silver deposited during electrolysis could be related to the number of electrons transferred and, subsequently, to NA. Another method involved using black-body radiation theory. More modern techniques utilize X-ray crystallography to determine the volume of the unit cell of a crystal, and then, by knowing the molar volume of the substance, Avogadro's number can be calculated.
The significance of Avogadro's number extends far beyond just counting particles. It is essential for converting between mass and the number of atoms or molecules. For example, to determine the mass of a single atom of carbon, you would divide the molar mass of carbon (approximately 12 grams/mole) by Avogadro's number. This calculation gives you the mass of a single carbon atom in grams, providing a tangible connection between the macroscopic property of mass and the microscopic world of atoms.
Furthermore, Avogadro's number is crucial in stoichiometry, the branch of chemistry that deals with the quantitative relationships between reactants and products in chemical reactions. By using Avogadro's number, chemists can convert between the number of moles of reactants and products, allowing them to predict the amount of product formed or the amount of reactants needed for a complete reaction. This is essential in industrial chemistry for optimizing reaction yields and minimizing waste.
Trends and Latest Developments in Determining Avogadro's Number
In recent years, significant advancements have been made in refining the value of Avogadro's number. One of the most precise methods used today is the X-ray crystal density (XRCD) method, which involves measuring the lattice parameter and molar volume of highly pure silicon crystals. This method, employed by the Avogadro project, aimed to redefine the kilogram based on fundamental constants. By accurately determining the number of atoms in a known mass of silicon, scientists sought to link the kilogram to Avogadro's number and Planck's constant, moving away from the reliance on a physical artifact (the international prototype kilogram).
The motivation behind redefining the kilogram stemmed from the inherent instability of physical artifacts. Over time, the mass of the international prototype kilogram, stored at the International Bureau of Weights and Measures (BIPM) in France, was found to vary slightly compared to its official replicas. This uncertainty, although small, posed a challenge for precise measurements in science and technology. By basing the kilogram on fundamental constants, scientists aimed to create a more stable and universally accessible standard.
The Avogadro project involved growing highly pure, isotopically enriched silicon-28 crystals. These crystals were meticulously characterized for their isotopic composition, density, and lattice parameter. X-ray interferometry was used to measure the lattice parameter with extremely high precision. The molar volume was then calculated from the density and isotopic composition, allowing for a highly accurate determination of Avogadro's number.
While the redefinition of the kilogram based on fundamental constants has been successful, ongoing research continues to refine the value of Avogadro's number. Scientists are exploring new methods and improving existing techniques to reduce uncertainty and improve accuracy. One promising area of research involves using quantum metrology to develop new measurement techniques that can provide even more precise determinations of fundamental constants.
The impact of these advancements extends beyond just the redefinition of the kilogram. A more accurate value of Avogadro's number has implications for a wide range of scientific disciplines, including materials science, nanotechnology, and pharmaceuticals. It enables more precise measurements of atomic and molecular properties, leading to a better understanding of the fundamental laws of nature.
Tips and Expert Advice on Using Avogadro's Number
Avogadro's number can seem daunting at first, but with a few practical tips, you can master its use in various calculations. First, always remember the definition: 1 mole = 6.02 x 10^23 entities. This simple equation is the key to unlocking a wide range of problems in chemistry. Understanding the relationships between moles, mass, and the number of particles is essential for success in quantitative analysis.
When solving problems involving Avogadro's number, start by clearly identifying what you are given and what you need to find. Write down the known values and the units associated with them. Then, determine the appropriate conversion factors needed to convert between the given quantities and the desired quantity. For example, if you are given the mass of a substance and need to find the number of atoms, you will need to convert the mass to moles using the molar mass, and then convert moles to the number of atoms using Avogadro's number.
Pay close attention to units. Units are your best friend in chemistry, as they can help you track your calculations and ensure that you are using the correct conversion factors. Always include units in your calculations and make sure that they cancel out appropriately. For example, if you are converting from grams to moles, you will need to use the molar mass, which has units of grams per mole. By multiplying the mass in grams by the reciprocal of the molar mass (i.e., moles per gram), you can convert grams to moles.
Another useful tip is to use scientific notation correctly. Avogadro's number is a very large number, so it is typically expressed in scientific notation. Make sure you understand how to perform calculations with numbers in scientific notation. When multiplying or dividing numbers in scientific notation, remember to add or subtract the exponents, respectively. When adding or subtracting numbers in scientific notation, make sure they have the same exponent before performing the operation.
Don't be afraid to break down complex problems into smaller, more manageable steps. Sometimes, a problem may seem overwhelming at first, but by breaking it down into smaller steps, you can tackle each step individually and then combine the results to obtain the final answer. For example, if you are asked to calculate the mass of a certain number of molecules in a compound, you can first calculate the number of moles of the compound, then calculate the molar mass of the compound, and finally multiply the number of moles by the molar mass to obtain the mass of the compound.
Finally, practice, practice, practice! The more you practice solving problems involving Avogadro's number, the more comfortable you will become with the concepts and the calculations. Work through examples in your textbook, online resources, and practice problems provided by your instructor. Don't be afraid to ask for help if you are struggling with a particular concept or problem. Your instructor, teaching assistants, and classmates can be valuable resources for learning and understanding Avogadro's number.
FAQ About Avogadro's Number
Q: Who is Avogadro, and what was his contribution to chemistry?
A: Amedeo Avogadro was an Italian scientist who proposed Avogadro's Law in 1811, which states that equal volumes of gases at the same temperature and pressure contain the same number of molecules. While he didn't determine the exact value of Avogadro's number, his law laid the foundation for understanding the relationship between the number of particles and the volume of a gas.
Q: How is Avogadro's number determined?
A: Avogadro's number has been determined using various methods, including electrolysis, black-body radiation theory, and X-ray crystallography. The X-ray crystal density (XRCD) method, which involves measuring the lattice parameter and molar volume of highly pure silicon crystals, is one of the most precise methods used today.
Q: Why is Avogadro's number important?
A: Avogadro's number is essential for converting between mass and the number of atoms or molecules. It is also crucial in stoichiometry, the branch of chemistry that deals with the quantitative relationships between reactants and products in chemical reactions. It allows chemists to perform accurate quantitative analyses and predict reaction outcomes.
Q: What is a mole?
A: A mole is a unit of amount in chemistry that represents 6.02 x 10^23 entities (atoms, molecules, ions, etc.). Just as a "dozen" always means 12, a mole always means 6.02 x 10^23 entities.
Q: How do I use Avogadro's number in calculations?
A: To use Avogadro's number in calculations, start by clearly identifying what you are given and what you need to find. Write down the known values and the units associated with them. Then, determine the appropriate conversion factors needed to convert between the given quantities and the desired quantity. Pay close attention to units and use scientific notation correctly.
Conclusion
Avogadro's number, 6.02 x 10^23, is more than just a number; it's a fundamental constant that bridges the gap between the microscopic and macroscopic worlds, playing a vital role in quantitative chemical analysis. From its historical development through the contributions of scientists like Avogadro, Loschmidt, and Perrin, to its modern-day applications in stoichiometry and materials science, NA allows chemists to accurately measure and predict the behavior of matter. By understanding and applying Avogadro's number correctly, you can unlock a deeper understanding of chemical reactions and the composition of substances.
Ready to put your knowledge of Avogadro's number to the test? Try working through some practice problems, explore advanced topics in stoichiometry, or even delve into the research behind the redefinition of the kilogram. Share your experiences and questions in the comments below – let's continue the journey of discovery together!
Latest Posts
Latest Posts
-
Which Is More 2 3 Or 3 4
Nov 28, 2025
-
What Kinds Of Plants Are In The Rainforest
Nov 28, 2025
-
When Does Of Mice And Men Take Place
Nov 28, 2025
-
What Is The Monomer Of Nucleic Acids
Nov 28, 2025
-
Boudica Rise Of The Warrior Queen
Nov 28, 2025
Related Post
Thank you for visiting our website which covers about Avogadro's Number Is Equal To 6.02x1023 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.